Environmental NanoChemistry Lab (ENCL) at WUStL
Our group research aspiration is to solve important energy and environmental problems by applying a molecular-level understanding of the nanoscale formation and transformation of natural and engineered materials in solid–water interfacial systems.
We advance our understanding of environmental interfacial reactions by providing in situ, real-time quantitative and qualitative information from unique interdisciplinary experimental approaches. By providing crucial information for upscaling, our research particularly benefits the larger scale processes needed to make a major impact.
Based on our strong scientific understanding of nanoscale interfacial chemistry and solid nucleation, current research in the Jun Group (Environmental NanoChemistry Laboratory) focuses on the following research topics. Selected examples are given with links to videos or representative papers.
Improve Fundamental Understanding of Nanochemistry
-
Nucleation of Solids (carbonates, phosphates, sulfates, Li dendrites, and iron and manganese oxides)
-
Dissolution of Materials
-
Nanoscale Interfacial Structure Evolution
-
Nanoconfinement and Nanopores
Advance Engineered Systems for Sustainability:
-
CO2 capture, utilization, and sequestration
Watch the recording of the Jackson Award Lecture of the Clay Mineral Society in July 2022
-
Energy-related subsurface operations (Underground hydrogen storage and unconventional energy recovery)
-
Nutrient (P & N) and critical materials (Rare Earth Elements, Co, and Ni) recovery and reuse
Supercritical Fluid-Enhanced Selective Recovery of Rare Earth Elements
-
Hydrogel technology for environmental applications
Mineral-Hydrogel Composites for Managing Harmful Algal Blooms
-
Photothermal membrane development for water purification
-
Photochemically-enabled green chemistry for nanostructure synthesis
Photochemically-Assisted Fast Formation of δ-MnO2 Nanosheets
-
Managed aquifer recharge (Arsenic mobilization and water reuse)
-
Innovative water technologies
-
Reactive oxidizing species (reactive oxygen species, reactive halogen species, and alkyl radicals)
-
Scale formation and their prevention in desalination processes or pipelines
-
Biomineralization, biomaterials, and bio-inspired chemistry for novel materials development
We are experts who use various surface-sensitive techniques including synchrotron-based X-ray techniques and provide in-depth knowledge of water, surface, and solid chemistries.
Facility and Equipment
The Environmental NanoChemistry Laboratory is well-equipped with various water chemistry instruments, reverse osmosis membrane test bench reactor and a high pressure pump, nanoparticle synthesis setups including thermo-controlled systems, five sets of high-pressure and high-temperature controlled syringe pumps (Teledyne ISCO, Lincoln, NE) and Hastelloy C bench scale reactors (Parr Company, IL), and in situ SAXS microreactors for supercritical CO2 experiments. Our group also has two sets of high pressure and high temperature pH electrodes (Corr Instrument, TX), which enables us to monitor in situ pH conditions in the CO2–H2O-mineral systems.
For in situ microscopic observations of surface reactions under aqueous conditions, our group has installed an Atomic Force Microscopy (Veeco, Nanoscope V) with the capability of changing electrochemical conditions and temperatures (up to 60 oC). The AFM room is strictly isolated from sound and vibration in order to resolve atomic-scale surface changes. In addition, we possess an atmospheric chamber with catalyst fan boxes which can host the entire AFM setup, allowing various atmospheric- and temperature-controlled experiments for redox sensitive reactions.
To prepare a thin coating of organic matter, we use a Spin Processor (Laurell Technologies, PA) in our lab. Our lab also has a Contact Angle Analyzer (Phoenix 300, Surface Electro Optics Co. Ltd) for surface hydrophilicity/hydrophobicity and surface tension measurements and an Ion Chromatography (Dionex ICS-1600) for anions and organic acid concentration measurements. We also have a high pressure and temperature Fourier Transform Infrared Spectroscope (FTIR) and a UV/VIS spectroscope. For photochemical and photothermal tests, we utilize a 450W Xenon Arc lamp setup (Newport) and solar chamber (Xe-1-BC, Q-Lab Corporation) to simulate the sunlight exposure. We also utilize Raman Confocal Microscopy (Renishaw, U.K.) for solid phase identification.
For quantitative and qualitative analyses of experimental samples, we will utilize scanning electron microscopy (SEM)-Energy-dispersive X-ray spectroscopy (EDX) and SEM-Backscattered electron spectroscpy (BSE), regular and high resolution transmission electron microscopy (HR-TEM) from the Institute of Materials Science and Engineering (IMSE) and X-ray Fluorescence (XRF), Microprobe, and X-ray diffraction (XRD) from the Earth and Plenatary Science department. In addition, we use inductively coupled plasma-mass spectrometer (ICP-MS, 7500ce, Agilent Technologies, CA), ICP-optical emission spectrometry (OES), BET, total organic carbon (TOC) analyzer, gas chromatography-FID, and Zetasizer instrument (Dynamic Light Scattering and Zeta Potential Measurement, Nano ZS, Malvern Instruments Ltd.), at the Jens Molecular & Nanoscale Analysis Laboratory (Departmental Common Instrumental Facility) and Nano Reseach Facility (NSF funded common facility). To create standard nanoparticle deposted substrates with known coverages, we use a Physical Vapor Deposition (PVD) method.
One of our main experimental approaches is utilizing synchrotron-based X-ray techniques at the Advanced Photon Source (APS) at Argonne National Laboratory such as small angle X-ray scattering (SAXS), grazing incidence SAXS (GISAXS), wide angle X-ray scattering (WAXS) or high energy total scattering for pair distribution function analysis, and X-ray absorption spectroscopy (XAS). As of April 2023, we have earned 136 successful beamtime allocations at Synchrtron X-ray natioanl facilities.
For thermodynamic calculations, our group use Geochemists’ Workbench (GWB, Release 8.0, RockWare, Inc.), MINEQL+, and visual MINTEQ.
For reactive transport modeling, we have been collarboating with Dr. Carl I. Steefel at the Lawrence Berkeley National Lab and using CrunchTope to predict the contaminant and elemental transport aspects at multiscale.
The NAE Committee on Engineering's Grand Challenges has identified 14 areas awaiting engineering solutions in the 21st century. Please visit the national academy of engineering website for more detailed information.
Among 14 grand challenges, our research group actively involves in two areas: (1) Provide access to clean water and (2) Develop carbon sequestration methods.
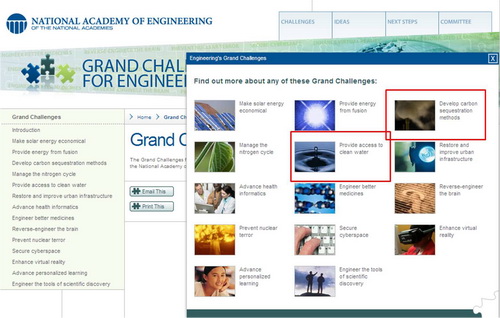 (figure source: The National Academy of Engineering)
(figure source: The National Academy of Engineering)
The goals of Environmental NanoChemistry Lab are well-aligned with the United Nations' Sustainable Development Goals (Figure source: The United Nations' Sustainable Development Goals)
(Figure source: The United Nations' Sustainable Development Goals)
Additional Instrumentation List
Institute of Materials Science and Engineering (IMSE)
National Nanotechnology Infrastructure Network (NNIN)
Funding Support
We gratefully acknowledge support from the following agencies and programs:
- International Center for Advanced Renewable Energy & Sustainability (I-CARES)
- Center for Materials Innovation (CMI)
- Consortium of Clean Coal Utilization (CCCU)
- McKelvey Scholarship
- Mr. and Mrs. Spencer T. Olin Fellowship
- Summer Undergraduate Research Fellowship
- Washington University Summer Engineering Fellowship (WUSEF)
- MAGEEP Undergraduate Research Fellowship
- McDonnell International Scholars Academy
- MARC U-STAR: Maximizing Access to Research Careers Undergraduate Student Training in Academic Research Fellowship
Extramural Funding Agencies and Companies
- American Chemical Society Petroleum Research Fund (ACS-PRF)
- NSF Environmental Chemical Sciences Program (CHE)
- NSF Gebiology and Low-Temperature Geochemistry program (EAR)
- NSF Biomaterials Program (DMR)
- NSF Environmental Engineering Program (CBET)
- NSF CAREER award
- Ralph E. Powe Junior Faculty Award, Oak Ridge Associated Universities
- U.S. EPA Environmental Protection Agency
- Shaw Environmental & Infrastructure Group, Inc.
- U.S. Department of Energy, Office of Basic Energy Sciences, Energy Frontier Research Center (EFRC)
- U.S. Department of Energy, Office of Basic Energy Sciences (DOE-BES)
- National Alliance for Water Innovation (NAWI)-U.S. Department of Energy's Energy Efficiency & Renewable Energy (DOE-EERE)
- National Institutes of Health (NIH)
- National Institute of Environmental Health Sciences (NIEHS)
- U.S. Department of Energy, National Energy Technology Laboratory (DOE-NETL)
- Committee on the Advancement of Women Chemists (COACh)
- Environmental Protection Agency (EPA) Science to Achieve Results (STAR) Fellowship
- NSF Graduate Research Fellowships Program (GRFP)
- NSF Graduate Teaching Fellows in K-12 Education (GK-12) program
- National Nanotechnology Infrastructure Network (NNIN)-Research Experience for Undergraduates (REU) Summer Program-Undergraduate researchers
- Students and Teachers as Research Scientists (STARS) Program-High school student researchers
