Course Instructor
| Environmental NanoChemistry (EECE 534) (Graduate Level Elective Course) |
Washington University | Spring 2008, 2010, 2011, 2012, 2013, 2014, 2016, 2018, 2020, 2021, 2023, 2024 | |
Aquatic Chemistry (EECE 505, previously EECE 543/443) (Graduate and Upper Level Undergraduate Course, and anchor course for engineered aquatic cluster qualification exam) |
Washington University |
Fall 2013, 2014, 2015, 2016, 2017, 2018, 2019, 2020, 2021, 2022, 2023 |
|
Engineering Analysis of Chemical Systems (ChE 351) (Chemical Engineering Undergraduate Core Course) |
Washington University |
Fall, 2008, 2009, 2010, 2011, 2012 |
|
Independent Study on Environmental NanoChemistry (for Both Undergraduate and Graduate Students) |
Washington University |
Fall 2008-present |
-
Student interviews
Jacqueline Rogers Who is your favorite instructor and why?
"Dr. Jun has been my favorite instructor because of her willingness to help her students truly understand the material. Dr. Jun also encouraged her students to work on skill sets important to graduate students, like written and oral communication and proposal writing, in a productive manner."
Duo Zhang Can you describe some of the projects you’ve worked on since earning your master’s degree?
"Fort Irwin is in the Mojave Desert. The Army wants to keep working in this zone, but they can’t without a source of clean water. Before they used recycled water, but now there is an irrigation system that handles potable water, non-potable water, green water and more. When I got here, the colonel in charge of the garrison asked me to put together a report on what improvements could be made to the water system. The data that was provided to me to review was from the 1980s. I worked with contractors and environmental officers to update the fort’s data and improve the models they were used in. Last summer, the secretary of the Army came to Fort Irwin and reviewed the report. She approved it, and we got $12 million to make the updates. I was prepared for this project by what I learned in Professor Young-Shin Jun's class."
-
EECE 505: Aquatic Chemistry (Graduate Level Elective Course and Upper Level Undergraduate Elective Course; Graduate Aquatic Cluster Core Course)
Aquatic chemistry governs aspects of the biogeochemical cycling of trace metals and nutrients, contaminant fate and transport, and the performance of water and wastewater treatment processes. This course examines chemical reactions relevant to natural and engineered aquatic systems. A quantitative approach emphasizes the solution of chemical equilibrium and kinetics problems. Topics covered include chemical equilibrium and kinetics, acid-base equilibria and alkalinity, dissolution and precipitation of solids, complexation of metals, oxidation-reduction processes, and reactions on solid surfaces. A primary objective of the course is to be able to formulate and solve chemical equilibrium problems for complex environmental systems. In addition to solving problems manually to develop chemical intuition regarding aquatic systems, software applications for solving chemical equilibrium problems are also introduced.
-
EECE 534: Environmental NanoChemistry (Graduate Level Elective Course and Upper Level Undergraduate Elective Course)
New course developed in 2008 (To the best of our knowledge, this course is one of the earliest courses about environmental nanochemistry and nanotechnology offered in the United States). This unique and innovative course involves the study of nanochemistry at various environmental interfaces, focusing on colloid, nanoparticle, and surface reactions. The course also (1) examines the thermodynamics and kinetics of nanoscale reactions at solid-water interfaces in the presence of inorganic or organic compounds and microorganisms; (2) investigate show nanoscale interfacial reactions affect the fate and transport of contaminants, including the environmental health and safety (EH&S) of nanotechnology; (3) introduces multidisciplinary techniques for obtaining fundamental information about the structure and reactivity of nanoparticles and thin films, and the speciation or chemical form of environmental pollutants at the molecular scale; and (4) explores connections between environmental nanochemistry and environmental kinetic analysis at larger scales. This course includes two-day lab classes, which provide opportunities to students to learn nanoparticle synthesis and its characterization using state of the art nanoscale sensitive techniques.
This course helps students attain a better understanding of the relationship between nanoscience/technology and the environment—specifically how nanoscience can potentially lead to better water treatments, more effective contaminated-site remediation, or new energy alternatives.
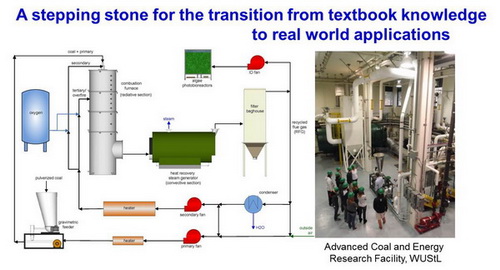
- ChE 351: Engineering Analysis of Chemical Systems, (Chemical Engineering Undergraduate Core Course: mainly sophomore)
Substantial revisions of existing course. The syllabus of the course was revised significantly to incorporate real environmental and chemical engineering examples, hand-on class demonstration, and the advanced energy facility at Washington University (tour and associated homework to check the students’ understanding of the new concepts)This course involves the use of mathematics and methods of engineering in analysis of chemical and physical processes in engineered and natural systems. It also teaches how to use and quantify balances and basic rate laws to describe mass and energy conservation in physical processes with and without chemical reaction.